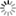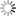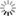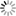Quantum Phenomena
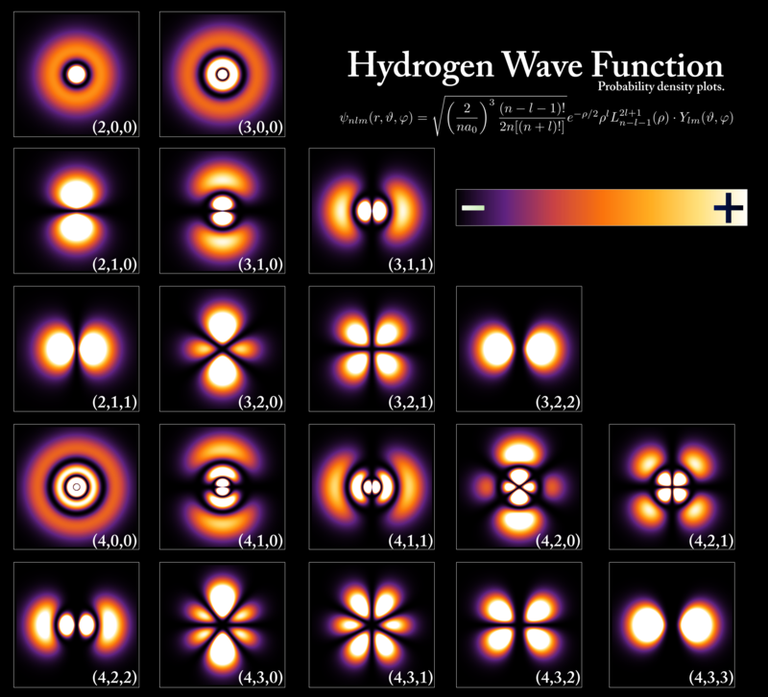
- [Hydrogen Wave Function - Wikipedia. (Wavefunctions of the electron in a hydrogen atom at different energy levels. Quantum mechanics cannot predict the exact location of a particle in space, only the probability of finding it at different locations.[1] The brighter areas represent a higher probability of finding the electron.)]
- Overview
Quantum phenomena are the peculiar behaviors of matter and energy at the microscopic level, such as wave-particle duality (particles acting as waves and vice versa), quantum superposition (a particle being in multiple states at once), and quantum entanglement (linked particles influencing each other instantly, regardless of distance).
Other key phenomena include quantum tunneling (particles passing through barriers), quantum interference (waves combining), and quantization (energy and other properties occurring in discrete packets). These effects are fundamental to technologies like lasers and transistors and are essential for understanding how the universe works.
1. Key Quantum Phenomena:
- Wave-Particle Duality: The concept that entities like electrons and photons can behave as both particles and waves, a contradiction in classical physics.
- Quantum Superposition: A system can exist in multiple states simultaneously until a measurement is made, which "collapses" it into a single state.
- Quantum Entanglement: Two or more quantum particles become linked, so that they share the same fate regardless of the distance separating them.
- Quantum Tunneling: A particle can "tunnel" through an energy barrier even if it doesn't have enough energy to overcome it classically.
- Quantum Interference: Like waves in water, quantum waves can interfere with each other, creating patterns of constructive and destructive interference.
- Quantization: Energy, momentum, and other properties of particles are not continuous but occur in discrete, indivisible units called quanta.
- Quantum Spin: An intrinsic quantum property of particles like electrons, which is not actually physical spinning but gives particles volume and structure.
2. Examples in Action:
- Fluorescent Lights: Electrons in mercury atoms jump to higher energy levels and then fall back down, emitting photons (light).
- Stars' Fusion: Quantum tunneling enables protons to overcome their electrical repulsion and fuse, powering the sun.
- Semiconductors: Quantum effects in materials are crucial for transistors, the building blocks of modern electronics.
3. Relevance to Everyday Life:
Although seemingly strange and counterintuitive, quantum phenomena are not limited to the subatomic world.
- All around us: These effects govern the stable structure of atoms, the elements, and all matter.
- Technology: Quantum principles are the basis for lasers, transistors, medical imaging (MRI), and potentially future quantum computers.